Pathology Services
Since 1971, EPL® has provided necropsy, histology, and pathology services trusted worldwide. With 20+ board-certified veterinary pathologists, we deliver timely, cost-effective support for investigative research, preclinical studies, and specialized services such as peer reviews and pathology working groups.
- Board-certified expertise across organ systems and species
- Research animals, medical devices, aquatic & avian models
- Interpreted to the latest published standards
- Ultrastructural investigations (EM)
Aquatic & Avian Pathology
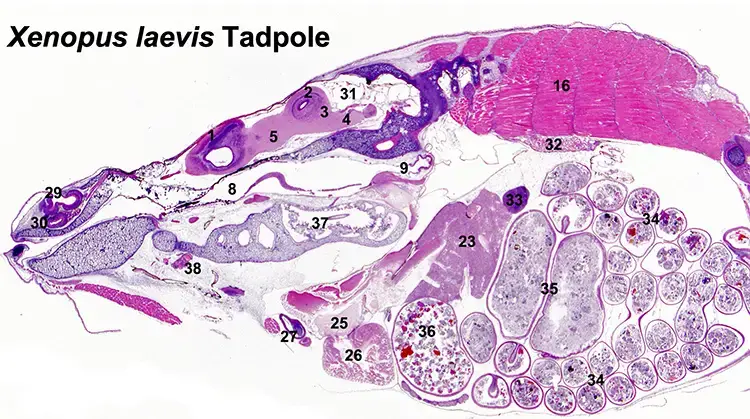
EPL offers specialized expertise in the post-mortem diagnostic evaluation of both laboratory and wild aquatic and avian species.
Our pathologists support toxicologic testing as well as environmental monitoring studies assessing the impact of xenobiotics on wildlife in natural ecosystems.In laboratory settings, we routinely evaluate commonly used fish models such as Japanese medaka, zebrafish, fathead minnows, rainbow trout, and amphibians like the African clawed frog and leopard frog . EPL has also contributed to field-based studies involving over thirty different species of freshwater, euryhaline, and marine fishes. Our avian pathology experience includes tissue preparation and evaluation in species such as Coturnix (Japanese) quail, domestic chickens and turkeys, and mallard ducks. These capabilities support a broad range of environmental toxicology, ecotoxicology, and regulatory testing applications.
Ask us more about our Aquatic & Avian Pathology Services
Carcinogenicity and Chronic Studies
Toxicology Pathology, Carcinogenicity and Chronic Studies
For Comprehensive Support for Carcinogenicity and Chronic Toxicology Studies, EPL offers expert support across all aspects of toxicologic pathology and carcinogenicity studies, including traditional rodent bioassays and the rasH2 transgenic mouse model. Our board-certified pathologists apply current diagnostic criteria with scientific precision, resulting in high-quality, predictive data to support regulatory decision-making.
We utilize Xybion’s Pristima® software for pathology data capture and maintain strong proficiency in SEND (Standard for Exchange of Nonclinical Data) compliance. Our team is also fully experienced with Instem’s Provantis™ system and can seamlessly enter pathology data directly into your preferred platform. Additionally, we offer annotated digital images tailored to your study requirements. Pathology reports can be customized to match your internal templates or delivered using EPL’s proven standard format. Whether you require full-service study support or targeted pathology expertise, EPL ensures your studies align with Good Laboratory Practice (GLP) guidelines as defined by the US FDA, US EPA, OECD, and other international regulatory authorities.
Contact us today to strengthen your carcinogenicity and chronic study programs with proven pathology expertise and regulatory compliance.
Organ/Therapeutic Expertise
Comprehensive Pathology Expertise Across Organ Systems and Study Types
EPL’s board-certified pathologists bring deep expertise across all organ systems and most animal species commonly used in discovery, investigative, and preclinical safety assessment studies. Many of our senior pathologists have served as co-authors of the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) publications, reflecting their leadership in toxicologic pathology standards.
Our team supports a wide range of therapeutic research areas and target-organ toxicity studies with unmatched scientific depth and regulatory understanding. Areas of pathology expertise include:
- Aquatic endocrine disruptor studies
- Cardiovascular system
- Dermal pathology
- Enhanced histopathology for developmental immunotoxicology
- Female reproductive system
- Fetal and neonatal histopathology (developmental evaluation)
- Hepatobiliary system
- Lymphoid system (thymus, lymph node, spleen, bone marrow)
- Male reproductive system
- Nervous system (central and peripheral)
- Ocular pathology
- Renal system
- Respiratory system
- Skeletomuscular system (bone and joints)
Inhalation Toxicology and Respiratory Tract Pathology EPL has extensive experience in inhalation toxicology studies and the evaluation of respiratory tract tissues in animals exposed to chemicals or test compounds via various exposure routes. Our pathologists understand the importance of precise tissue handling to preserve sensitive structures for evaluation of treatment-related effects. We are proficient in all common inhalation exposure models, including:
- Nose-only exposure
- Whole-body exposure
- Intra-nasal instillation (droplets or spray)
- Intra-tracheal and laryngeal aspiration
During necropsy, our technical team ensures that lung tissues are instilled with fixative at consistent pressure, preserving alveolar architecture for accurate histopathologic assessment. We also follow optimized methods for processing nasal passages, larynx, and lungs to capture site-specific effects often seen in inhalation studies. EPL pathologists have authored over 150 scientific papers, abstracts, book chapters, and presentations related to toxicologic pathology, including seminal reviews on nasal and laryngeal toxicity. Our team also led the development of two widely referenced CD-ROMs on Respiratory Tract Collection in Dogs and Rodents, which outline best practices for histological preparation of respiratory tissues.
Partner with EPL for expert pathology support across preclinical and regulatory studies, from whole-body evaluations to complex organ-specific investigations.
Pathology Working Groups

Independent Peer Review by Expert Panels.
EPL is a nationally and internationally recognized leader in organizing and conducting Pathology Working Groups (PWGs)—a specialized form of peer review used to address complex or controversial findings in toxicologic pathology studies. Unlike standard peer review, which is conducted by a single pathologist, PWGs bring together a panel of expert pathologists to deliver an independent, consensus-based interpretation of pathology data.PWGs are particularly valuable in situations that involve:
- Diagnostic uncertainty or disagreement between pathologists
- Studies with regulatory impact or agency interest
- Pivotal studies with controversial or high-stakes endpoints
- Comparisons of data from multiple laboratories or studies
- Mediation of interpretive discrepancies in final reports
EPL’s PWG methodology and procedures are documented in the widely referenced book chapter “Peer Review and Pathology Working Groups” (Mann and Hardisty, 2013), co-authored by EPL pathologists Dr. Peter Mann and Dr. Jerry F. Hardisty. This chapter serves as a key reference on the purpose, procedures, and regulatory importance of PWGs and includes recommended literature to further support toxicologic pathology evaluations.
PWGs are not routine components of every GLP or toxicologic study, but they are critical tools for resolving specific diagnostic questions or regulatory concerns. Independent reviews by a qualified expert panel can significantly strengthen study conclusions and provide assurance to regulatory authorities. EPL has unmatched experience in organizing and facilitating Pathology Working Groups for a wide variety of sponsors, study types, and regulatory contexts. Our expertise ensures a rigorous, transparent, and scientifically defensible review process.
Contact EPL to learn how a Pathology Working Group can support your study’s success and provide clarity in complex or high-impact pathology evaluations.
Pathology Peer Review

The landscape of pathology is evolving rapidly with advancements in Whole Slide Imaging (WSI) integration and the growing adoption of digital workflows.
While these technologies promise to streamline peer reviews and reporting, the transition can pose challenges. At EPL, we offer a proprietary peer review platform that’s ready to bridge the gap, providing unmatched flexibility, expertise, and independence.Why Choose Our Peer Review Platform?
- Works Seamlessly with Any Reporting System.Whether your lab uses Pristima, Provantis, or any other reporting system, our solution integrates effortlessly. Our platform is system-agnostic, allowing you to maintain your existing workflows without compromise.
- Glass or Digital: Your Choice.The shift to Whole Slide Imaging (WSI) is undeniable, with many in the industry increasingly adopting digital pathology for primary reads and peer reviews. However, glass slides continue to play a vital role in numerous workflows. Our platform supports both formats, enabling seamless peer reviews without disruption, regardless of your current setup.
- WSI Expertise You Can Count On.Managing WSI transfers can be complex, but it’s an area where we excel. With years of experience handling large-scale WSI transitions and integrations, we ensure data integrity, security, and usability every step of the way.
- Independent and Unbiased.Unlike built-in peer review modules that may tie you to specific systems, our platform operates independently. This independence ensures your lab retains full control over its peer review processes while benefiting from our cutting-edge technology and expertise.
Preparing for the Future
The rise of WSI and the integration of peer review modules in pathology systems are reshaping how labs operate. Our platform not only helps you navigate these changes but positions your lab at the forefront of innovation. Whether you’re fully digital, using glass slides, or transitioning to a hybrid model, we’ve got you covered.Discover how EPL can enhance your peer review workflows with independence, flexibility, and reliability.
Research Animal Models
Expert Pathology Support for Preclinical Animal Models
EPL’s board-certified pathologists bring extensive experience and scientific insight to studies involving commonly used animal models in biomedical research. We offer collaborative support for both standard and specialized models, helping to ensure accurate data interpretation and regulatory readiness.Animal models play a critical role in understanding disease mechanisms and evaluating new therapies prior to human trials. Our pathologists are well-versed in the evaluation and interpretation of pathology data generated from a wide range of in vivo research studies. By leveraging EPL’s expertise, you can reduce the risk of repeat studies, minimize animal use, and generate high-quality, reproducible data. We support a variety of disease-specific animal models, including but not limited to:
- Diabetic Nephropathy Models:5/6 nephrectomy, genetic rodent models (e.g., db/db, DBA/2J mice), unilateral ureteral obstruction
- Pulmonary Arterial Hypertension Models:Monocrotaline, SU5416/hypoxia, hypoxia
- Pulmonary Fibrosis Models:Bleomycin-induced fibrosis in rodents.
Contact us to discuss how EPL can support your animal model-based research with accurate pathology interpretation and GLP-compliant reporting.
Transmission Electron Microscopy
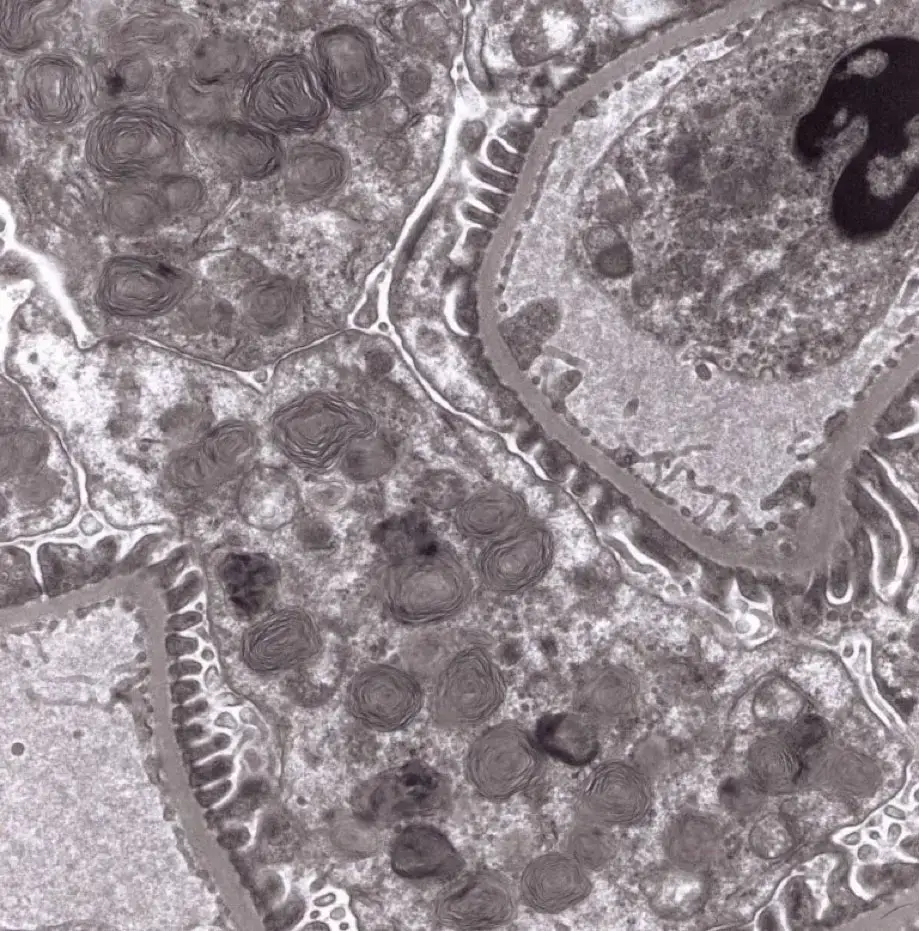
Transmission Electron Microscopy
For Transmission Electron Microscopy (TEM) in non-GLP and Research Studies EPL provides advanced transmission electron microscopy (TEM) services through collaboration with the Bioscience Electron Microscopy Laboratory (BEML) the University of Connecticut. The BEML offers comprehensive capabilities for ultrastructural analysis.- Tissue trimming and fixation
- Processing and epoxy resin embedding
- Sectioning for light and electron microscopy
- Imaging and interpretation of ultrastructural data
Whether for preclinical research or regulatory toxicology, EPL ensures a seamless integration of TEM services into your study workflow.
Contact EPL to incorporate GLP-compliant transmission electron microscopy into your next research or regulatory study—backed by decades of experience and scientific precision.
